A 2.32 g sample of Na2SO4 x nH2O yields 1.42 g Na2SO4 upon heating. Na2SO4 x nH20(s) yields Na2SO4(s) + nH20(g) What is the value of n? Group of answer choices
Phase diagrams of Na2SO4–MgSO4–CO(NH2)2–H2O system at 60 °C and their application – ScienceDirect
2+ Downloaded Solutions Melbourne, Australia Mostly Asked From Molar mass / g-mol! Na2SO4 142 PQ-19. A 2.32 g sample of Na2SO4’nH20 yields 1.42 g Na2SO4 upon heating. Na2SO4.nH2O (s) Na2SO4 (s) + nH2O (g) What is the value of n? (A) 2 (B) 3 (C) 5 (D) 10 Expert’s Answer Solution.pdf Next Previous Related Questions Q:

Source Image: youtube.com
Download Image
Chemistry I Question Subject: Chemistry

Source Image: chemistry.stackexchange.com
Download Image
na2so4.10h2o sodium sulphate decahydrate| Alibaba.com Determine molecular formula using the Ideal Gas Law Hydrate lab calculations Examples and Problems only, no solutions Here’s a worksheet with eight hydrate problems, all of which have (hand-written) solutions. The original location of this document is

Source Image: youtube.com
Download Image
A 2.32 G Sample Of Na2so4 Nh2o
Determine molecular formula using the Ideal Gas Law Hydrate lab calculations Examples and Problems only, no solutions Here’s a worksheet with eight hydrate problems, all of which have (hand-written) solutions. The original location of this document is Science Chemistry A 2.32g sample of Na2SO4*nH2 yields 1.42g Na2SO4 upon heating. What is the value of n? Na2SO4*nH20—>Na2SO4 (s)+nH20 (g) Molar Mass Na2SO4 =142 g/mol Molar Mass H2O = 18.016 g/mol A 2.32g sample of Na2SO4*nH2 yields 1.42g Na2SO4 upon heating. What is the value of n?
A 7.66 g sample of hydrated sodium sulphate, `Na_(2)SO_(4). xH_(2)O`, forms 4.06g of anhydrous – YouTube
May 6, 2023answered • expert verified A 2.32g sample of Na2SO4*nH2 yields 1.42g Na2SO4 upon heating. What is the value of n? thesultan8334 is waiting for your help. Add your answer and earn points. Add answer +10 pts Expert-Verified Answer No one rated this answer yet — why not be the first? 😎 tarabscorium The value of n in Na2SO4*nH2O is 5. na2so4.10h2o sodium sulphate decahydrate| Alibaba.com

Source Image: alibaba.com
Download Image
Ex situ XRD patterns of P2-type Na 0.67 Mn 0.67 Ni 0.33Àx Mg x O 2… | Download Scientific Diagram May 6, 2023answered • expert verified A 2.32g sample of Na2SO4*nH2 yields 1.42g Na2SO4 upon heating. What is the value of n? thesultan8334 is waiting for your help. Add your answer and earn points. Add answer +10 pts Expert-Verified Answer No one rated this answer yet — why not be the first? 😎 tarabscorium The value of n in Na2SO4*nH2O is 5.

Source Image: researchgate.net
Download Image
Phase diagrams of Na2SO4–MgSO4–CO(NH2)2–H2O system at 60 °C and their application – ScienceDirect A 2.32 g sample of Na2SO4 x nH2O yields 1.42 g Na2SO4 upon heating. Na2SO4 x nH20(s) yields Na2SO4(s) + nH20(g) What is the value of n? Group of answer choices

Source Image: sciencedirect.com
Download Image
na2so4.10h2o sodium sulphate decahydrate| Alibaba.com Chemistry I Question Subject: Chemistry

Source Image: alibaba.com
Download Image
Hóa chất Sodium Sulfate Anhydrous Ar – Na2SO4 – 500g – 497-19-8 – Xilong Dec 13, 2022A 2.32 g sample of Na2SO4 · nH2O yields 1.42 g Na2SO4 upon heating: Na2SO4 · nH2O(s) ↑ Na2SO4(s) + nH2O(g). What is the value of n? Molar mass of Na2SO4 = 142 g/mol. (B) (C) (D) 10. Close . Submitted by Jennifer B. Sep. 07, 2021 07:21 p.m. Video Answer. Solved by verified expert

Source Image: tschem.com.vn
Download Image
Sodium Phosphate Dibasic Dodecahydrate (Na2HPO4.12H2O) Molecular Weight Calculation – Laboratory Notes Determine molecular formula using the Ideal Gas Law Hydrate lab calculations Examples and Problems only, no solutions Here’s a worksheet with eight hydrate problems, all of which have (hand-written) solutions. The original location of this document is
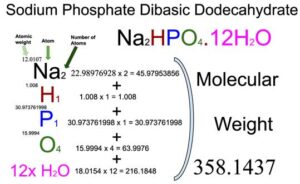
Source Image: laboratorynotes.com
Download Image
Phase Diagram for the Na2SO4–(NH4)2SO4–MEA–H2O System at Elevated Temperature | Journal of Chemical & Engineering Data Science Chemistry A 2.32g sample of Na2SO4*nH2 yields 1.42g Na2SO4 upon heating. What is the value of n? Na2SO4*nH20—>Na2SO4 (s)+nH20 (g) Molar Mass Na2SO4 =142 g/mol Molar Mass H2O = 18.016 g/mol A 2.32g sample of Na2SO4*nH2 yields 1.42g Na2SO4 upon heating. What is the value of n?

Source Image: pubs.acs.org
Download Image
Ex situ XRD patterns of P2-type Na 0.67 Mn 0.67 Ni 0.33Àx Mg x O 2… | Download Scientific Diagram
Phase Diagram for the Na2SO4–(NH4)2SO4–MEA–H2O System at Elevated Temperature | Journal of Chemical & Engineering Data 2+ Downloaded Solutions Melbourne, Australia Mostly Asked From Molar mass / g-mol! Na2SO4 142 PQ-19. A 2.32 g sample of Na2SO4’nH20 yields 1.42 g Na2SO4 upon heating. Na2SO4.nH2O (s) Na2SO4 (s) + nH2O (g) What is the value of n? (A) 2 (B) 3 (C) 5 (D) 10 Expert’s Answer Solution.pdf Next Previous Related Questions Q:
na2so4.10h2o sodium sulphate decahydrate| Alibaba.com Sodium Phosphate Dibasic Dodecahydrate (Na2HPO4.12H2O) Molecular Weight Calculation – Laboratory Notes Dec 13, 2022A 2.32 g sample of Na2SO4 · nH2O yields 1.42 g Na2SO4 upon heating: Na2SO4 · nH2O(s) ↑ Na2SO4(s) + nH2O(g). What is the value of n? Molar mass of Na2SO4 = 142 g/mol. (B) (C) (D) 10. Close . Submitted by Jennifer B. Sep. 07, 2021 07:21 p.m. Video Answer. Solved by verified expert